Abstract
Background:
A histologic finding of large cell transformation (LCT) in Mycosis fungoides (MF) is often associated with an aggressive clinical course and inferior prognosis (Arulogun et al. Blood 2008). In patients (pts) with advanced MF (stage IIB-IV), LCT has been established as an independent prognostic factor (Scarisbrick et al. JCO 2015). Although CD30 expression is observed more frequently in MF with LCT vs without LCT, a wide range of CD30 expression levels is observed in LCT lesions and the level of expression lacks prognostic value for MF (Vergier et al. Blood. 2000). The ALCANZA study (NCT01578499) demonstrated significantly better rates of objective response lasting ≥4 months (ORR4) (∆43.8%; p<0.0001) and progression-free survival (PFS) (16.7 months vs 3.5 months; p<0.0001) with brentuximab vedotin vs physician's choice (PC) of oral methotrexate or bexarotene in adults with previously treated CD30+ MF or primary cutaneous anaplastic large-cell lymphoma (Prince et al. Lancet 2017). Despite variability in CD30 expression, significant improvements in ORR4 and PFS were consistently seen with brentuximab vedotin over PC, across all CD30 expression levels in pts with MF. This post-hoc analysis characterized the proportion of pts with LCT, efficacy of brentuximab vedotin in pts with LCT and relationship to CD30 expression.
Methods:
Analyses were performed on skin biopsies taken from 98 pts with previously treated CD30+ MF who were randomized 1:1 to receive brentuximab vedotin or PC. CD30 expression levels were measured by immunohistochemistry on samples obtained during screening of pts for the ALCANZA study. LCT status at baseline was assessed using ≥2 biopsies obtained at screening; pts were deemed to have LCT if any biopsy showed presence of LCT (large cells - with nuclei ≥4 times larger than those of normal lymphocytes - present in >25% of total dermal infiltrate or forming microscopic nodules). Objective response rates lasting ≥4 months (ORR4), PFS, and safety endpoints were assessed according to LCT and CD30 expression.
Results:
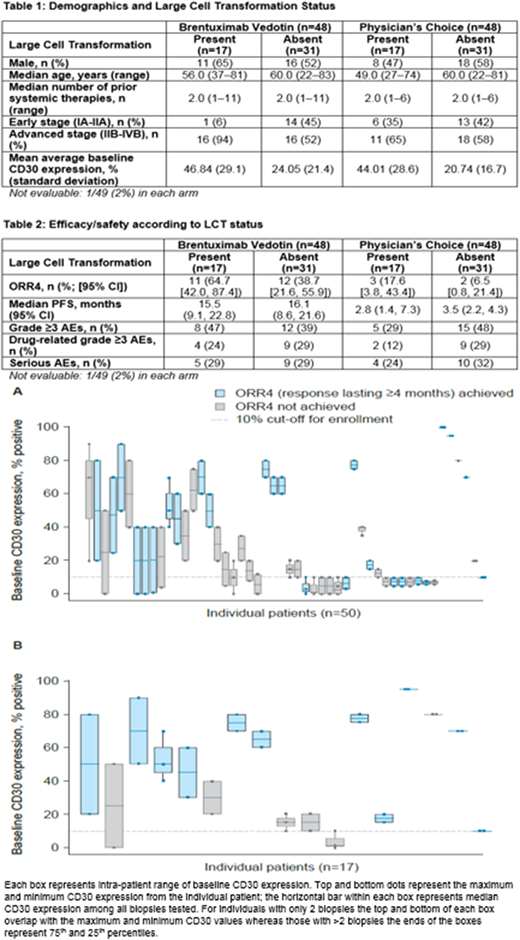
Baseline demographics and LCT status by disease stage are described in Table 1. In both arms, 17/49 pts (35%) pts had LCT, and LCT was found more frequently in stage IIB pts (59% [brentuximab vedotin] and 41% [PC]). Brentuximab vedotin improved ORR4 vs PC in pts with LCT (11/17 [64.7%] vs 3/17 [17.6%]; p=0.006) and without LCT (12/31 [38.7%] vs 2/31 [6.5%]; p=0.003) (Table 2). Median PFS improved with brentuximab vedotin vs PC in pts with LCT (15.5 vs 2.8 months; HR=0.304; 95% CI 0.139, 0.668; p=0.002) and without LCT (16.1 vs 3.5 months; HR=0.364; 95% CI 0.200, 0.662; p<0.001). Median PFS follow-up times were 26.0 months (both arms, pts without LCT), 36.0 months (brentuximab vedotin arm, pts with LCT), and not estimable (PC arm, pts with LCT). Grade ≥3 adverse event (AE), drug-related grade ≥3 AE and serious AE rates were similar across LCT status groups (Table 2), and CD30 expression levels and were not associated with treatment-emergent AEs in the brentuximab vedotin arm.
In pts with LCT, median average CD30 expression was 50% of total dermal infiltrate (range 3-95%) in the brentuximab vedotin arm and 35% (6.3-97.5%) in the PC arm (Figure 1), although pts with high baseline average CD30 expression (upper tercile of all enrolled MF pts) were more likely to have LCT; in the high CD30 expression sub-group, 56% and 64% of pts had LCT in the brentuximab vedotin and PC arms, respectively. Globally, pts with LCT treated with brentuximab vedotin who achieved an ORR4 had higher median average baseline CD30 levels (65%) vs pts who did not attain ORR4 (20%). Of the pts with LCT who achieved an ORR4, 9/11 pts in the brentuximab vedotin arm had baseline average CD30 expression levels ≥40%, but responses were also noted in the 2 pts with low average CD30 levels (10% and 17.5%).
Conclusions:
Previously, there were limited data on the relationship between LCT status and clinical outcome or CD30 expression in MF pts treated with brentuximab vedotin. Our analyses demonstrated that pts with MF benefitted from brentuximab vedotin regardless of LCT status. A wide range of CD30 expression levels was observed in pts with MF and LCT. In these pts, high baseline CD30 expression levels were generally predictive of a good response to treatment with brentuximab vedotin although meaningful responses were observed in those with lower CD30 levels.
Kim:Merck: Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tetralogic: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; miRagen: Research Funding; Medivir: Membership on an entity's Board of Directors or advisory committees; Kyowa-Kirin-Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Horizon Pharma: Consultancy, Research Funding; Portola: Research Funding; Soligenix: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galderma: Research Funding; Forty Seven Inc: Research Funding; Neumedicine: Consultancy, Research Funding. Prince:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Whittaker:Galderma: Research Funding. Horwitz:Trillium: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Spectrum: Research Funding; Infinity/Verastem: Consultancy, Research Funding; Kyowa-Hakka-Kirin: Consultancy, Research Funding; Aileron Therapeutics: Consultancy, Research Funding; Forty Seven: Consultancy, Research Funding; Portola: Consultancy; Mundipharma: Consultancy; Innate Pharma: Consultancy; Seattle Genetics: Consultancy, Research Funding; Corvus: Consultancy. Duvic:UT MD Anderson Cancer Center: Employment; Shape: Research Funding; Concert Pharmaceuticals, Inc.: Consultancy; Forty Seven, Inc.: Membership on an entity's Board of Directors or advisory committees; Precision Oncology, LLC: Membership on an entity's Board of Directors or advisory committees; Aclaris Therapeutics Int'l Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; MEDACorp: Consultancy; Cell Medica Inc.: Consultancy, Honoraria; Medscape: Other: Speaker/Preceptor; Array Biopharma: Consultancy, Honoraria; American Council on Extracorporeal Photopheresis (ACE): Membership on an entity's Board of Directors or advisory committees; Medivir AB: Membership on an entity's Board of Directors or advisory committees; Spatz Foundation: Research Funding; Rhizen Pharma: Research Funding; Dr. Reddy's Laboratories (A.K.A. Promius Pharma): Consultancy; Mallinckrddt Pharmaceuticals (formerly Therakos): Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kiniksa Pharmaceuticals: Consultancy; The Lynx Group: Consultancy; MiRagen Therapeutics: Consultancy; Guidepoint Global: Consultancy; Soligenix, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Huya Bioscience Int'l: Consultancy; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Evidera, Inc.: Consultancy; Huron Consulting Group: Consultancy; Defined Health: Consultancy; Taiwan Liposome Company LTD: Consultancy; Jonathan Wood & Associates: Other: Speaker; Celgene Corp: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Eisai: Research Funding; Allos: Research Funding; Oncoceuticals: Research Funding; Tetralogics: Research Funding; Kyowa Hakko Kirin, Co: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Clinical Care Options: Consultancy. Bechter:MSD: Consultancy; Pierre Fabre: Consultancy; Sanofi: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy. Stadler:ICN: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy; Millennium Pharmaceuticals, Incmited: Consultancy; Johnson and Johnson: Membership on an entity's Board of Directors or advisory committees. Scarisbrick:Takeda harmaceuticals: Consultancy. Zinzani:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Eradat:Novartis: Research Funding; Celgene: Research Funding; Gilead: Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Pharmacyclics: Research Funding. Ortiz-Romero:Actelion: Consultancy; Innate Pharma: Consultancy; 4SC: Consultancy; Takeda: Consultancy; MEDA: Research Funding; Patent of PLG1 mutation for diagnostic or treatment of cutaneous lymphomas: Patents & Royalties: Patent of PLG1 mutation for diagnostic or treatment of cutaneous lymphomas; Janssen: Other: Travel Expenses; Roche: Other: Travel Expenses; Abbvie: Other: Travel Expenses. Akilov:Kyowa Kirin: Consultancy; Seattle Genetics: Consultancy; Pfizer: Research Funding; Trillium Therapeutics: Research Funding; Actelion Pharmaceuticals: Consultancy. Trotman:PCYC: Research Funding; Jassen: Research Funding; Celgene: Research Funding; Roche: Research Funding; Janssen: Research Funding; Beigene: Research Funding. Weichenthal:Takeda: Consultancy; Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; TEVA: Other: Grant. Fisher:Genetech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics Inc.: Membership on an entity's Board of Directors or advisory committees. McNeeley:Quest Diagnostics: Employment, Equity Ownership. Gru:Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Wang:Seattle Genetics, Inc.: Employment, Equity Ownership. Palanca-Wessels:Seattle Genetics, Inc.: Employment, Equity Ownership. Lisano:Seattle Genetics: Employment, Equity Ownership. Li:Seattle Genetics, Inc.: Employment. Lin:Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Little:Takeda: Employment, Equity Ownership. Trepicchio:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Dummer:Bristol-Myers Squibb (BMS): Consultancy, Membership on an entity's Board of Directors or advisory committees; Sun Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pierre Fabre: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck Sharp & Dhome (MSD): Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal